 |
Veterinary Anesthesia & Analgesia Support Group |
| Practical Information for the Compassionate Veterinary Practitioner |
|
| HOME |
|
 |
||||
|
|||||
|
|||||
| Myofascial Trigger Points | |||||
| Rick Wall, DVM | |||||
| Center for Veterinary Pain Management and Rehabilitation, The Woodlands, TX | |||||
| 4-2014 | |||||
David Simons, MD, a well-known expert in muscle pain and dysfunction in people, and coauthor of Travell and Simons’ – Myofascial Pain and Dysfunction -The Trigger Point Manual, referred to muscle as an “orphan organ”. He said, “No medical specialty claims it. As a consequence, no medical specialty is concerned with promoting funded research into the muscular causes of pain, and medical students and physical therapists rarely receive adequate primary training in how to recognize and treat myofascial trigger points." In dogs skeletal muscle makes up approximately 44% of live body weight in mixed breed and purebred dogs. In greyhounds muscle to live weight is approximately 57%.1 However, there continues to be a deficiency in both veterinary education and veterinary literature, about skeletal muscle and its role in pain and dysfunction. Muscle pain (Myalgia) Muscle pain or myalgia in people is described as an aching, cramping pain that is difficult to localize and can be referred to deep somatic tissues. Myalgia activates unique cortical structures and is inhibited more strongly by descending pain-modulating pathways. Activation of muscle nociceptors is much more effective at inducing neuroplasticity in the dorsal horn neurons that occur in chronic pain.2 Myalgia strongly activates the anterior cingulate cortex and periaqueductal gray (PAG). These areas are known to be associated with emotions in people and depression often accompanies chronic myalgic conditions such as fibromyalgia. These areas of the brain are not affected by cutaneous pain.3,4 Muscle pain often follows joint injury or dysfunction. If the joint is not functioning properly mechanical stress can be placed on the functional unit muscles of that joint (flexors, extensors, adductors, abductors, etc.). However, muscle pain and dysfunction can also lead to joint dysfunction. Muscle pain can also follow nerve injury or dysfunction. Muscles innervated by an irritated or injured nerve can become painful. Myalgia is defined as pain in a muscle or muscle group. Myalgia in dogs may result from;
Overuse of muscle can result in muscle injury and myalgia. This type injury is commonly defined as muscle strain. Muscle strain has been defined as acute distraction injury of muscles and tendons.5 However, in people the term “strain” is used with a high degree of variability between practitioners and it is the author’s opinion the same is true in veterinary medicine.6 To address this variability a new comprehensive classification system was recently developed for people.6 Functional muscle disorders – acute indirect muscle disorder without macroscopic evidence of muscle tear
Structural muscle injury – any acute indirect muscle injury with macroscopic evidence of muscle tear
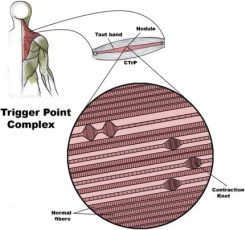
Inflammatory myopathies in dogs have been described as a heterogeneous group of disorders characterized by nonsupprative cellular infiltration of skeletal muscle.7 Polymyositis would be classified as a generalized inflammatory myopathy whereas masticatory muscle myositis and extraocular myositis are classified as focal. Clinical signs include muscle atrophy, muscle weakness, and in most cases myalgia. Muscle weakness and myalgia have been reported in dogs associated with hypoglycemia, hyperglycemia, electrolyte disturbances, hypothyroidism, hypoadrenocorticism, hyperadrenocorticism, and renal failure.8 Nutritional deficiencies, more properly termed insufficiencies, have been reported in people as a cause of myalgia.9,10,11 At present, veterinary literature has not reported myalgia or other pain as clinical signs associated insufficiencies. Muscle Plasticity Skeletal muscle is one of the most adaptable (plastic) tissues in the body. It is well recognized that the main reason change is created in skeletal muscle is the level of muscle activity relative to normal.12 Every structural aspect of muscle can change given the proper stimulus. Muscle plasticity is an important consideration in change in muscle that occurs due to injury and disease. Plasticity is also important in training and conditioning of the canine athlete. Myofascial trigger points (MTPs) A myofascial trigger point can be defined as a hyperirritable spot located within a taut band in skeletal muscle.13 The presence of MTPs within muscle can be a source of pain and dysfunction in dogs; however, limited information exists in veterinary literature. The emergence of the disciplines of veterinary rehabilitation and sports medicine demand a better understanding of the role MTPs play in muscle pain and dysfunction. Characteristics of Myofascial Trigger Points MTPs have three major characteristics, sensory, motor and autonomic. The muscle pain or myalgia associated MTPs is described in humans as diffuse, deep and difficult to localize with defined referred pain patterns. When the MTP is stimulated manually a localized pain is appreciated. Very often palpation of a MTP in dogs can result in a “jump sign”, a pain response resulting in vocalization and/or withdrawal as pressure is applied. Other sensory aspects include peripheral and central sensitization. Peripheral sensitization can be described as a reduction in threshold and an increase in responsiveness of the peripheral ends of nociceptors. Central sensitization is best explained as a physiochemical change resulting in an increase excitability of neurons within the central nervous system.9 Motor abnormalities of MTPs include the development of a taut band within the muscle, a local twitch response (LTR) with stimulation, muscle weakness without atrophy and loss of reciprocal inhibition.14 The taut band is a localized, linear, discrete band of hardened muscle within the softer homogeneous muscle. Taut bands are identified running parallel to muscle fibers and can be described as a localized contracture within the muscle without nerve-initiated activation of the motor endplate or neuromuscular junction.15 In contrast to the taut band is muscle spasm, the latter is the result of increased neuromuscular tone of the entire muscle due to a nerve-initiated contraction. A muscle spasm that is painful is referred to as a muscle cramp. The contracture associated with the taut band also results in reduction in range of motion. The MTP is located within the taut band and is what distinguishes it from other painful areas with in muscle.16 The local twitch response (LTR) is another motor component of MTPs. The LTR is a unique involuntary spinal cord reflex resulting in a rapid contraction of the taut band following manual stimulation of the MTP. Manual stimulation can be accomplished by direct palpation or introduction of a needle. The LTR in dogs can also serve as verification of the presence of a MTP. In people weakness is recognized in muscles that have MTPs. This weakness occurs without atrophy and is not related to neuropathy or myopathy.13 Weakness is usually rapidly reversible immediately upon inactivation of the MTP. This rapid reversal suggests weakness is caused by inhibition of muscle activation. It is also recognized that a MTP in one muscle can inhibit effort or contractile force in another muscle suggesting a central inhibition process.16 Additional motor or muscle dysfunction from MTPs is brought about by disordered recruitment or altered muscle activation patterns in muscles that work together to produce a specific action.16,17 Reciprocal inhibition is defined as inhibition of antagonist muscle contraction during contraction of agonist muscle. This central inhibition of muscle activity results in coordinated quality movement. Reciprocal inhibition becomes reduced when agonist or the activated muscle contains a MTP resulting in co-contraction and subsequent altered gait and decreased quality of movement. In dogs, simple tests can be performed to evaluate muscle weakness. With a dog in standing position, slowly slide limb backward until non-weight bearing. A slight to profound drop of the contralateral side can be indicative of muscle weakness and/or altered muscle firing patterns associated with MTPs within the anti-gravity muscles of that limb. Sit to stand exercise can serve as a subjective assessment of weakness by observation of dog’s ability to both sit and stand. Video analysis of gait patterns and quality of gait may be helpful when pre and post therapy videos are compared. Etiology of Myofascial Trigger Points Trigger points are composed of contraction knots that can be described as segments of muscle fiber with intensely contracted sarcomeres and increased diameter. It remains unclear why these contraction knots form however the hypothesis, known as the Integrated Trigger Point Hypothesis postulates a problem at the motor endplate resulting in excessive release of acetylcholine. This excess of acetylcholine results in sarcomere shortening that has been observed histopathologically.18 Contraction knots or areas of concentrated focal sarcomere contraction have been described in animals and humans.19 A better appreciation of the potential causes or etiologies of MTP formation in dogs will aid the clinician in both recognition and therapy. Muscle-related mechanisms associated with the development of MTPs, in people and animals; include muscle overuse or overload and direct trauma. Low-level muscle contractions, uneven intramuscular pressure distribution, direct trauma, unaccustomed eccentric contractions, eccentric contraction in unconditioned muscle, and maximal or submaximal concentric contractions can lead to muscle injury and subsequent development of MTPs.9,20 The Integrated Trigger Point Hypothesis, in 1981, was the first scientific hypothesis to explain the formation of taut bands and MTPs based on both electrophysiological and histopathological data.21 The hypothesis postulates that muscle injury leads to increased calcium concentrations outside the sarcoplasmic reticulum, possibly due to mechanical rupture of the sarcoplasmic reticulum or the sarcolemma. Increased calcium concentrations result in sustained muscle fiber contraction. This hypothesis was later refined, in 2004, to include a dysfunctional motor endplate occurring secondary to muscle injury and resulting in excessive release of acetylcholine (ACh).22 Sustained maximal contraction of the muscle fibers in the vicinity of the dysfunctional endplate causes increased metabolic demand and decreased concentrations of adenosine triphosphate (ATP). The calcium pump that returns calcium to the sarcoplasmic reticulum is ATP dependent, as is the un-crosslinking of actin and myosin, thus calcium concentrations and contractile activity remain increased. Muscle injury alters the normal equilibrium between the release and breakdown of ACh and its removal by acetylcholinesterase from acetylcholine receptors in the postsynaptic membrane. Substances such as calcitonin gene related peptide (CGRP) and Substance P (SP), released during muscle injury, facilitate increased release of ACh, inhibition of breakdown, and upregulation of acetylcholine receptors.22 A persistent muscle fiber contraction develops leading to the development of the taut band and subsequent MTP. Perpetuating Factors in the Development of Myofascial Trigger Points Perpetuation of MTP formation in dogs is most often due to mechanical stresses resulting in chronic muscle overload. Postural changes in the dog due to orthopedic injury, postoperative surgical trauma and pain, neuropathy, joint dysfunction, and pain related to osteoarthritis create muscle overload. Many of the same muscle-related mechanisms that lead to development of MTPs also perpetuate them. Chronic osteoarthritis creates compensatory postural changes that activate and perpetuate MTPs in numerous muscles. Moderate to severe osteoarthritis of the coxofemoral joints activates and perpetuates MTPs in the functional unit muscles of the coxofemoral joint, flexors (including iliopsoas), adductors, and extensors. The cranial shift in weight overloads muscles in the thoracic limbs, namely the m. infraspinatus, m. deltoideus, and the long head of the m. triceps brachii. Repeated lateral flexion of the spine, which assists in ambulation by advancing the pelvis and pelvic limb while limiting coxofemoral flexion and extension, results in overloading of the m. iliocostalis umborum. Another clinical example is the dog with a non-weight bearing pelvic limb lameness that adopts hopping actions in the contralateral pelvic limb resulting in unaccustomed eccentric contractions of the coxofemoral and stifle extensors in an attempt to limit flexion. Lumbar paraspinal muscles become overloaded, as they must now assist with ambulation and not just spinal stabilization. The m. iliopsoas, which is actually two separate muscles, the m. psoas major and m. iliacus, develops MTPs, contracture, often resulting in a kyphotic posture. In people additional perpetuating factors are identified, nutritional, metabolic, nerve Hypothyroidism, a metabolic and endocrine disorder, is recognized in people as a perpetuating factor for MTP. Clinical signs for people include myalgia, stiffness, weakness, cramps, and pain upon exertion.13 Hypothyroidism is the most common endocrine disorder in dogs and is associated with a variety of clinical signs; however, the veterinary literature does not mention pain as a consequence of hypothyroidism. In people visceral pain can activate and perpetuate MTPs in the area of referred pain. Neurons in the dorsal horn of the spinal cord receive input from the viscera and from receptors in the skin and deeper soft tissues.23 As a result of this overlap, visceral nociceptive activation of the dorsal horn neurons may result in muscle pain and may also be a cause of MTPs in animals. Examination for Myofascial Trigger Points Examination of muscles for myalgia is not part of the standard physical, orthopedic, or neurologic examination in veterinary medicine. Identification of taut bands and the hypersensitive MTP within muscle is an acquired skill that requires and understanding of these changes, skilled instruction, and repeated practice. There are two basic palpation techniques employed in a myofascial examination: Flat Palpation: Examination by finger pressure that proceeds across the muscle fibers at a right angle to their length while compressing them against a firm underlying structure, such as a bone. This technique could be used for the m. infraspinatus, m. upraspinatus, and m. psoas major. Pincer Palpation: Examination of a part of a muscle by holding it in a pincer grasp between the thumb and fingers. Groups of muscles fibers are rolled between the tips of the digits to detect taut bands. This technique could be used for the m. triceps, m. sartorius, and m. tensor fascia latae. Myalgia can be appreciated in individual muscles with examination of the patient in the standing position. However, taut bands and MTPs are easier to appreciate in a relaxed muscle by placing patient non-weight bearing position such as lateral recumbency. In each position, an assistant is needed to provide gentle patient restraint because examination can induce a jump sign. Education of the client prior to the myofascial examination is needed to avoid concern when pain is elicited. Treatment of Myofascial Trigger Points Treatment of myofascial trigger points (MTPs) in dogs and cats consists of noninvasive and invasive therapies. Currently, there are no studies to validate the effectiveness of any therapy in either, and reported results remain strictly anecdotal. In people, numerous studies exist regarding noninvasive and invasive MTP therapy; however, in many the diagnosis of myofascial pain syndrome may lack validity. One literature review, reported significant variability in criteria used to diagnose myofascial pain syndrome and MTPs in people.24 Noninvasive MTP Therapy Therapeutic Lasers: Lasers are a popular modality that has been heavily marketed for the treatment of pain and wounds in the veterinary patient. Class IIIa (3a) lasers provide a maximum output power of 5 milliwatts (mW), class IIIb lasers provide output power up to 500 mW, and Class IV lasers provide output power greater than 500 mW. Several Class IV lasers are marketed for veterinary use with power output ranging from 1 watt to 15 watts. The amount of laser energy delivered during a treatment session is reported in Joules (J), and one Joule is equal to 1 watt per 1 second. The dose is reported as the energy per session in Joules divided by the area (cm2) where the energy is directed; therefore, the therapeutic laser dose is indicated in Joules/cm2. Accurate therapeutic dose delivery is only possible with Class IIIa and IIIb but not for Class IVs. The later must be continually moved over the treatment area to prevent thermal injury, therefore, the joules delivered per cm2 is only an estimate. Total laser energy delivered to the target tissue within the patient becomes even more uncertain due to beam reflection and back-scatter (collectively referred to as remittance). For the above reasons studies in people, regarding myofascial pain, are limited to Class IIIs. Treatment with Class IIIa and Class IIIb lasers is more commonly referred to in the literature as low-level laser therapy (LLT). Low-level laser therapy has been widely used in the treatment of MTPs in people. Several double-blind placebo controlled studies report positive effects of LLT on MTPs.25,26,27 However, other studies report no therapeutic benefit.28,29 One systemic review did conclude that LLT could be effective in the treatment of MTPs associated with lateral epicondylitis in people.30 Proper therapeutic dosages for treatment are not known, and conflicting information exists in humans and in animal models. It has been suggested that inadequate dosage may be the cause of the unpredictability in the reported efficacy of laser therapy, especially by those marketing Class IV lasers.26 However, two studies have reported efficacy with a lower dosage.26,30 The later study, using the rabbit as an animal model, reported better treatment outcomes with energy of 5.4J per session versus 14.4J per session (total energy for 5 sessions was 32.4J and 86.4J, respectively). Electrotherapies: Several references exist that discuss the use of transcutaneous electrical nerve stimulation (TENS) in the management of pain in dogs; however, no specific mention of its use in myofascial pain was found.31,32,33 TENS combined with other physical modalities appeared to have an immediate effect with regard to decreasing myofascial pain in people.34 However, others concluded that insufficient evidence is available to determine the effectiveness of TENS in myofascial pain.35 Therapeutic Ultrasound: One randomized controlled study reported an immediate reduction in MTP sensitivity with therapeutic ultrasound in humans.36 Therapeutic ultrasound has been used to decrease stiffness of latent MTPs in the trapezius muscle in people.37 In that study, a 3 MHz therapeutic ultrasound was used at 1.4 Watts/cm2 for 5 minutes in a circular motion on an area twice the size of the 7cm2 ultrasound sound head. This is in contrast to previous studies that reported therapeutic ultrasound was no more effective than placebo.38,39 Gam et al, surveyed patients up to 6 months after treatment by means of a patient questionnaire, while studies reporting benefits from therapeutic ultrasound were based on immediate patient response only. Physical/Manual Therapies: Data is either inadequate or conflicting regarding most manual therapies for treatment of myofascial pain syndrome.40 Another references states, current evidence regarding treatment of MTPs with physical and manual therapies did not exceed the moderate level of evidence.35 It was additionally asserted that most trials examined multimodal treatment programs, so positive effects cannot exclusively be credited to a particular therapy. Ischemic compression, also known as trigger point pressure release, is a commonly described manual therapy for the treatment of MTPs in people. Studies in people show that ischemic compression may be of benefit in treatment of MTPs associated with shoulder pain, neck pain, headaches, and carpal tunnel syndrome.34,41,42,43 Numerous descriptions of the technique can be found in the academic medical literature as well as in the lay literature regarding massage therapy. Digital compression of the MTP for 60 to 90 seconds with increasing pressure is the most commonly described method. In dogs the taut band is identified and examined for the exquisitely tender MTP then digital pressure is applied to the point of patient recognition. After 15 to 20 seconds pressure may gradually be increased in most patients. Providing a gentle stretch to the muscle while applying pressure may assist in release of the MTP. Invasive MTP Therapy Several types of invasive MTP therapy have been described in people, including trigger point dry needling with an acupuncture needle and MTP injections with local anesthetics and other substances. In the author’s experience, MTP injections are not well tolerated by the veterinary patient. Dry needling (DN) can be defined as a skilled intervention that uses a thin solid filament needle to penetrate the skin and stimulate underlying myofascial trigger points for the treatment of myofascial pain and muscle dysfunction. The American Physical Therapy Association defines DN as an invasive technique used by physical therapists (where allowed by state law) to treat myofascial pain that uses a dry needle, without medication or injection, which is inserted into areas of the muscle known as trigger points.44 Similarities exist between DN and the Traditional Chinese Medicine style of acupuncture, however, there are also very significant differences. Acupuncture follows rules and beliefs developed from ancient times, whereas DN ignores ancient acupuncture philosophy. DN is based more on modern scientific neurophysiology and anatomy.45 DN has more in common with Western Medical Acupuncture (WMA) since this form of acupuncture is a more modern scientific approach to therapy. WMA still uses mostly predetermined point for needle placement many of which were developed from evaluation of traditional Chinese acupuncture. Neurophysiology of Western Medical Acupuncture The placement of acupuncture needles in the tissue produces therapeutic effects through stimulation of peripheral nervous system and can be divided into four categories local, segmental, heterosegmental and general. Local effects: Local effects are brought about by antidromic stimulation of high threshold afferent nerves. This antidromic (conduction of an impulse in an axon opposite of normal directions) leads to release of trophic and vasoreactive substances within the local area of stimulation. Increased circulation is perhaps one of the most important local factors from needling. Segmental effects: When the needle is inserted into tissue changes can occur within the dorsal horn of the spinal cord. Within the laminal II, substantia gelatinosa, enkephalinergic interneurones inhibit C fiber pain transmission thus modulating nociception.46 Heterosegmental effects: Placing a needle anywhere in the body can produce actions that result in analgesia. Both the spinal cord and brain process the afferent input from the nociceptive stimulus of the needle. The central nervous system produces a descending pain inhibition and two such pathways have been described. A third analgesic system, induced by noxious stimulation anywhere in the body, is described as Diffuse Noxious Inhibitory Control (DNIC).47 A recent study, however, suggests that the low pain stimulus usually used in people may not produce sufficient noxious stimulation to produce DNIC.48 General effects: These are more difficult to define, and there is clearly some overlap with heterosegmental effects. The latter term is used here to denote effects mediated at every segment of the spinal cord, as opposed to effects mediated by humeral means or by influence on higher centers in the CNS controlling general responses.49 Trigger Point Dry Needling Further expansion of a DN definition, using the information above; Dry needling (DN) can be defined as a skilled intervention that uses a thin solid filament needle to penetrate the skin and stimulate underlying myofascial trigger points for the treatment of myofascial pain and muscle dysfunction. Local effects of needling appear to be due to the production of a local twitch response (LTR), but, segmental, heterosegmental and general effects maybe produced thus enhancing the management of pain and dysfunction. Dry needling has been shown to produce short-term anti-nociceptive effects.53 This effect was limited to muscles innervated by the same spinal cord segment. When a MTP in the infraspinatus was needled, pain thresholds to pressure were increased in MTPs in the supraspinatus (segments C5 and C6) while no change in pain threshold to pressure was found in MTPs in the gluteus medius (segments L4, L5, and S1). Pressure pain thresholds have also been shown to increase in active MTPs located in the trapezius of people demonstrated that pressure point thresholds in active MTPs in the trapezius with dry needling of MTrPs located in the extensor carpi radialis longus.54 Improvement in the range of motion of the neck was an additional finding. The management of pain and improvement in the active joint range of motion was seen following DN of MTPs in the scapulohumeral muscles of elite female athletes.55 The clinician who undertakes invasive therapy for the treatment of MTPs needs not only a thorough knowledge of anatomy, but also must develop the kinesthetic skills to accurately place the needle into the MTP. Employing the examination techniques previously described, the taut band is identified and its length examined to localize the MTP. The acupuncture needle (Seirin J Type No. 5 [0.25] with insertion tube) is rapidly inserted, with the aid of the insertion tube, into the superficial tissues and then directed into the deep tissues and muscle to the taut band. An appreciation of an increase in resistance as the needle enters the taut band develops with experience. If needle placement is accurate, a LTR may be appreciated in the taut band. The needle is moved in and out of the MTP, slowly, until no further LTRs are appreciated. In dogs, the LTR confirms the presence of an MTP, but does not distinguish between an active MTP and a latent MTP. Two landmark studies have helped to validate invasive MTP dry needling and the therapeutic importance of the local twitch response (LTR).56,57 After induction of the LTR by the needle entering the MTP, local concentrations of biochemical mediators such as Substance P and Calcitonin Gene Related Peptide decreased. This may explain the observed decrease in pain in people after release of the MTP. In the later study, not only were there similarities in the biochemical milieu of the active MTPs, but increased concentrations of analytes were found in remote muscle sites that did not contain MTPs. Study participants with active MTPs in the trapezius had increased concentrations of inflammatory mediators, neuropeptides, catecholamines, and cytokines in the gastrocnemius, which did not contain MTPs. The cause of this is not completely understood, however, it could be related to central sensitization. There is also the possibility that widespread release of these analytes is a precursor to the development of MTPs. Both studies offer explanations of the proposed therapeutic benefits of invasive therapeutic intervention and the secondary hyperalgesia that is often present in people with myofascial pain syndrome. The Canine Athlete Muscular injury can occur from a single or recurrent episodes of biomechanical overloading. Injured muscles are usually abnormally shortened with increased tone and tension due to varied states of over contraction and contracture.58 In people, myofascial pain, characterized by the presence of myofascial trigger points (MTPs), is estimated to account for 85% of muscle pain (myalgia) due to injury.13 Myofascial pain and MTPs are now being recognized as clinical entities in veterinary patients, however, minimal literature exists.59,60,61,62,63 With the exception of spontaneous pain there is no difference between active and latent MTPs. The later only produces pain when stimulated; however other MTP characteristics remain the same. MTPs produce muscle weakness, thought to be due to central inhibition. The muscle contracture brought about by the development of taut bands shortens muscle length and reduces joint range of motion. Altered muscle activation patterns and loss of reciprocal inhibition of antagonist muscles can directly affect coordinated limb and body movement.16,64 More recent findings in people suggest accelerated muscle fatigue and overloading of active muscle motor units close to the MTPs. Combined or alone these MTP characteristics potentiate decreased performance in the athlete. In elite swimmers it has been suggested that MTPs play a role in the development of shoulder pain.65 Range of movement and strength were improved and pain was reduced following treatment of MTPs in elite female volleyball players.66 The Washington Redskins and four other NFL teams embrace dry needling of MTPs. Elliott Jermyn, Redskins assistant athletic trainer, “Once the trigger point has been eradicated, one of the things we usually see right away is an increased range in motion, and increase in muscle production. The effectiveness is immediate, and there’s no doubt that these guys like them.”67 The etiology of muscle injury in the canine athlete can be caused by eccentric muscle contractions, maximal concentric muscle contractions, and unaccustomed muscle contraction; poorly conditioned canine athletes maybe at greater risk, especially those with poor core strength. Core body strength can be defined as the balanced development of muscles that stabilize, align and move the trunk of the body. In dogs this is primarily the dorsal and ventral paraspinal muscles and to a less degree the abdominal muscles. As previously mentioned muscle strain is poorly defined highly variable term used to describe muscle injury and pain. The more comprehensive classification system for muscle injury by European sports medicine physicians may more clearly define muscle injury.6 Functional muscle disorders – acute indirect muscle disorder without macroscopic evidence of muscle tear
Structural muscle injury – any acute indirect muscle injury with macroscopic evidence of muscle tear
In the canine athlete functional muscle disorders appear more common. However, canine athletes are more often treated empirically, without benefits of advanced imagining and/or musculoskeletal ultrasound that could define a structural problem. Muscle injury resulting in the development of taut bands and MTPs would be defined as a functional muscle disorder. Myalgia and/or muscle dysfunction due to muscle injury can usually be improved with the treatment of MTPs. It is the author’s opinion that treatment of latent MTPs may improve performance of the canine athlete: however, there are no current studies, in dogs, to validate this statement. Two of the more commonly reported muscles injured in the canine athlete are the iliopsoas (formed by the joining of the psoas major and the iliacus near its insertion on the lesser trochanter of the femur) and the teres major. The iliopsoas is very important core strength muscle. It is the author’s opinion that many canine athletes lack sufficient core strength to achieve optimum performance thus increasing risk of injury. The teres major aids in flexion of the shoulder, draws the humerus caudally and medially rotates the shoulder preventing lateral rotation.1 It is likely injured during rapid lateral movements while attempting to stabilize the shoulder. Just below the scapula the teres major joins the latissimus dorsi. Some injuries classified as teres major may actually be latissimus dorsi or a combination of both. The Orthopedic and Neurologic Patient The development of myalgia and muscle dysfunction in the orthopedic patient can be the result of mechanical stress resulting in muscle overload. Changes in posture, compensatory movements of ambulation, and altered limb loading are likely the etiologies related to the development of MTPs. The neurologic patient is similar with the addition of MTP development in muscles innervated by irritated nerves and/or spinal cord segments. In the non-weight bearing pelvic limb patient MTPs can develop in the muscles of contralateral limb (rectus femoris, vastus group. gluteals, biceps femoris, semitendinosus) due to eccentric contractions brought about during hopping movement. Continuous hip flexion maintains nonweight bearing resulting in low-level muscle contractions in the flexors of the hip, including the iliopsoas. MTPs develop in these muscles creating contracture and limiting coxofemoral joint range of motion often accompanied by a kyphotic posture. The later can be due to MTP development in the iliopsoas. These functional problems in the muscle can exist long after the initial orthopedic structural problem resolves. |
|||||
| Return to top of page | |||||
| Page References: | |||||
1. Evans HE, de Lahunta A: Miller’s Anatomy of the Dog, 4th Edition. Elsevier Saunders, 2013, St. Louis, Missouri 2. Dommerholt J, Shah JP: “Myofascial Pain Syndrome” In: Ballantyne JC, Rathmell JP, Fishman SM, Editors: Bonica’s Pain Management, 4th Edition, Lippincott, Williams & Wilkins, 2010. Baltimore, Maryland. Chapter 35:450-471 3. Svensson P, Minoshima S, Beydoun A, et al: Cerebral processing of acute skin and muscle pain in humans. J Neurophysiol 1997; 78(1):450-460 4. Niddam DM, Chan RC, Lee SH, et al: Central modulation of pain evoked from myofascial trigger point. Clin J Pain 2007; 23(5): 440-448 5. Hagglund M, Walden M, Bahr R, et al: Methods of epidemiological study of injuries to professional football players: developing the UEFA model. Br J Sports Med 2005; 39:340-346 6. Mueller-Wohlfahrt HW, Haensel L, Mithoefer K, et al: Terminology and classification of muscle injuries in sport: the Munich consensus statement. Br J Sports Med 2013; 47(6):342-350 7. Evans J, Levesque D, Shelton GD: Canine inflammatory myopathies: a clinicopathologic review of 200 cases. J Vet Intern Med 2004; 18(5):679-691 8. Shelton GD: Routine and specialized laboratory testing for the diagnosis of neuromuscular diseases in dogs and cats. Vet Clin Path 2010; 39(3):278-295 9. Dommerholt J, Huijbregts P: Myofascial Trigger Points – Pathophysiology and Evidence-Informed Diagnosis and Management 2011; Jones and Bartlett Publishers,Sudbury, Massachusetts 10. Gerwin R: A study of 96 subjects examined for both fibromyalgia and myofascial pain. J Musculoskel Pain 1995; 3:121 11. Gerwin R: A review of myofascial pain and fibromyalgia: Factors that promote their persistence. Acupunct Med 2005; 23:121-134 12. Lieber RL: Skeletal Muscle Structure, Function, and Plasticity – The Physiological Basis of Rehabilitation, 3rd Edition 2010; Lippincott Williams & Wilkins, Baltimore, Maryland and Philadelphia, Pennsylvania 13. Simons DG, Travell JG, Simons LS: Myofascial Pain and Dysfunction: The Trigger Point Manual. Volume 1. Upper Half of Body 1999; Lippincott Williams & Wilkins, Baltimore, Maryland 14. Simons DG, Stolov WC: Microscopic Features of Transient Contraction of Palpable Bands in Canine Muscle. Am J of Physical Med 1776; 55(2):65-88 15. Gerwin RD, Shannon S, Hong CZ, et al: Interrater reliability in myofascial trigger point examination. Pain 1997; 69:65-73. 16. Mense S, Gerwin RD: Muscle Pain: Diagnosis and Treatment 2010; Springer-Verlag Berlin Heidelberg. 17. Lucas KR, Rich PA, Polus BI: Muscle Activation patterns in the scapular positioning muscles during loaded scapular plane elevation: The effects of Latent Myofascial Trigger Points. Clin Biomech 2010; 8:765-70 18. Simons DG. (2004) Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol 2004; 14:95-107 19. Bron C, Dommerholt J: Etiology of myofascial trigger points. Curr Pain Headache Rep 2012; 16(5):439-444 20. Gerwin R: Myofascial pain syndrome: here we are, where we must go? J Musculoskeletal Pain 2010; 18:329-347 21. Simons DG, Travell JG: Myofascial trigger points, a possible explanation. Pain 1981; 10:106-109 22. Gerwin RD, Dommerholt J, Shah JP: An Expansion of Simon’s Integrated Hypothesis for Trigger Point Formation. Current Pain and Headache Rep 2004; 8:468-475 23. Gerwin RD: Myofascial and Visceral Pain Syndromes: Visceral-Somatic Pain Representations. J Musculoskel Pain 2002; 10(1/2):165-175 24. Tough EA, White AR, Richards S, et al: Variability of criteria used to diagnose myofascial trigger point pain syndrome – evidence from a review of the literature. Clinical Journal of Pain 2007; 23(3):278-286 25. Hakguder A, Birtane M, Gurcan S, et al: Efficacy of low level laser therapy in myofascial pain syndrome: An algometric and thermographic evaluation. Lasers in Surgery and Medicine 2003; 33:339-343 26. Gur A, Sarac AJ, Cevik R, et al: Efficacy of 904nm gallium arsenide low-level laser therapy in the management of pain in the neck: A double-blind and randomize-controlled trial. Lasers in Surgery and Medicine, 35:229-235 27. Ilbuldu E., Cakmak A, Disci R, et al: Comparison of laser, dry needling, and placebo laser treatments in myofascial pain syndrome. Photmedicine and Laser Surgery 2004; 22:306-311. 28. Altan L, Bingol, U, Aykac M, et al: Investigation of the effect of GaAs laser therapy on cervical myofascial pain syndrome. Rheumatology International 2005; 25(1):23-7. 29. Dundar U, Eveik D, Samili F, et al: The effect of gallium arsenide aluminum laser therapy in the management of cervical myofascial pain syndrome: A double blind, placebo-controlled study. Clinical Rheumatology 2007; 26:930-934 30. Chang WD, Wu JH, and Jiang JA: Therapeutic effects of low-level laser on lateral epicondylitis from differential interventions of Chinese-Western medicine: systematic review. Photomed Laser Surg, 28(3):327-36 31. Steiss JE: Muscle Disorders and Rehabilitation in Canine Athletes. Veterinary Clinics of North America: Small Animal Practic 2002 32(1):267-285 32. Mlacnik E, Bockstahler B, Muller M, et al: Effect of caloric restriction and moderate or intense physiotherapy program for treatment of lameness in overweight dogs with osteoarthritis. Journal of the American Veterinary Medical Association 2006; 229:1756-1760. 33. Canapp DA: Select modalities. Clinical Techniques in Small Animal Practice 2007; 22(4):160-165 34. Hou CR, Tsai LC, Cheng KF, et al: Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Archives of Physical Medicine and Rehabilitation 2002; 83(10):1406-1414 35. Dommerholt J, Huijbregts P: Myofascial Trigger Points – Pathophysiology and Evidence-Informed Diagnosis and Management 2011; Jones and Bartlett Publishers,Sudbury, Massachusetts 36. Aguilera FJ, Martin DP, Masanet RA, et al: Immediate effect of ultrasound and ischemic compression techniques for the treatment of trapezius latent myofascial trigger points in healthy subjects: a randomized controlled study. Journal of Manipulative Physiology and Therapy 2009; 32(7):515-520 37. Draper DO, Mahaffey C, Kaiser D, et al: Thermal ultrasound decreases tissue stiffness of trigger points in upper trapezius muscles. Physiotherapy Theory and Practice 2010; 26(3):167-172 38. Gam AN, Warming S, Larsen LE., et al: Treatment of myofascial trigger-points with ultrasound combined with massage and exercise—a randomized controlled trial. Pain 1998 77(1):73-79 39. Lee JC, Lin DT, Hong C: The effectiveness of simultaneous thermotherapy with ultrasound and electrotherapy with combined AC and DC current on the immediate pain relief of myofascial trigger points. Journal of Musculoskeletal Pain 1997 5:81-90 40. Mense S, Gerwin RD: Muscle Pain: Diagnosis and Treatment 2010; Springer-Verlag Berlin Heidelberg 41. Hains G, Descarreaux M, Hains F: Chronic Shoulder pain of myofascial origin: a randomized clinical trial using ischemic compression therapy. Journal of Manipulative and Physiological Therapy 2010; 33(5):362-369 42. Hains G, Descarreaux M, Lamy AM, et al: A randomized controlled (intervention) trial of ischemic compression therapy for chronic carpal tunnel syndrome. Journal of the Canadian Chiropractic Association 2010; 54(3):155-163 43. Montanez-Aguilera FJ, Valtuena-Gimeno N, Pecos-Martin D, et al: Changes in a patient with neck pain after application of ischemic compression as a trigger point therapy. Journal of Back and Musculoskeletal Rehabilitation 2010; 23(2):101-104 44. Physical Therapists & the Performance of Dry Needling – An Educational Resource Paper. Produced by the APTA Department of Practice and APTA State Government Affairs January 2012 45. Amaro JA:When Acupuncture Becomes “Dry Needling”. Dynamic Chiropractic 2008; 26(12) 46. Bowsher D: Mechanisms of acupuncture. In: Filshie J, White A, Editors. Medical Acupuncture – A Western Scientific Approach. First edition. Edinburgh: ChurchillLivingstone 1998; 69-82 47. Le Bars D, Dickenson AH, Benson JM: Diffuse noxious inhibitory control (DNIC). IEffects on dorsal horn convergent neurons in the rat; II – Lack of effect on nonconvergent neurons, supraspinal involvement and theoretical implications. Pain 1979; 6:305-327 48. Schliessbach J, van der Klift E, Siegenthaler A, Arendt-Nielson L, Curatolo M, Streitberger K: Does Acupuncture Needling Induce Analgesic Effects Comparable to Diffuse Noxious Inhibitory Controls? Evidence-Based Complementary and Alternative Medicine 2012; 785613 49. Dommerholt, Jan; Fernández de las Peñas, César: Trigger Point Dry Needling: An Evidence and Clinical-Based Approach (Kindle) 2013; Churchill Livingstone. KindleEdition 50. Furlan A.D, van Tulder M, Cherkin D, et al: Acupuncture and dry-needling for low back pain: an updated systemic review within the framework of the Cochrane collaboration. Spine 2005; 30(8):944-963 51. Tough EA, White A.R, Cummings TM, et al: Acupuncture and dry needling in the management of myofascial trigger point pain: A systematic review and meta-analysis of randomized controlled trials. European Journal of Pain 2009; 13:3-10 52. Fernandez-Carnero J, La Touche R, Ortega-Santiago R, et al: Short-term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disorders. Journal of Orofacial Pain 2010; 24(1):106-112 53. Srbely JZ, Dickey JP, Lee D, et al: Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. Journal of Rehabilitation Medicine 2010; 42(5):463-468 54. Tsai CT, Hsieh LF, Kuan TS, et al: Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. American Journal of Physical Medicine and Rehabilitation 2010; 89(2):133-140 55. Osborne NJ, Gatt IT: Management of shoulder injuries using dry needling in elite volleyball players. Acupuncture Medicine 2010; 28(1):42-45 56. Shah JP, Phillips TM, Danoff JV, et; alAn in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. Journal of Applied Physiology 2005 99:1977-1984 57. Shah JP, Danoff JV, Desai MJ, et al: Biochemicals Associated With Pain and Inflammation are Elevated in Sites Near to and Remote From Active Myofascial Trigger Points. Archives of Physical Medicine and Rehabilitation 2008; 89:16-23 58. Wheeler AH: Myofascial pain disorders: theory to therapy. Drugs 2004;64(1):45-62 59. Janssens LA: Trigger points in 48 dogs with myofascial pain syndromes. Veterinary Surgery 1991; 20(4):274-8 60. Janssens LA: Trigger point therapy. Problems in Veterinary Medicine 1992; 4(1):117-124 61. Simons DG, Stolov WC: Microscopic Features of Transient Contraction of Palpable Bands in Canine Muscle. Am J of Physical Med 1776; 55(2):65-88 62. Macgregor J, Graf von Schjweinitz D: Needle electromyographic activity of myofascial trigger points and control sites in equine cleidobrachialis muscle-an observational study. Acupunct Med 2006; 24(2):61-70 63. Wright B: Management of Chronic Soft Tissue Pain. Topics in Companion Animal Medicine 2010 25(1):26-31. 64. Lucas KR, Rich PA, Polus Bl: Muscle activation patterns in the scapular positioning muscles during loaded scapular plane elevation: the effects of Latent Myofascial Trigger Points. Clin Biomech 2010; 25(8):765-70 65. Hidalgo-Lozano A, Fernandez-de-las-Penas C, Calderon-Soto C, et al: Elite swimmers with and without unilateral shoulder pain: mechanical hyperalgesia and active/latent muscle trigger points in neck-shoulder muscles. Scand J Med Sci Sports 2013; 23(1):66-73 66. Osborne NJ, Gatt IT: Management of shoulder injuries using dry needling in elite volleyball players. Acupunct Med 2010; 28(1):42-45 67. Redskins’ Dry Needles Speed Recovery Time. http://www.redskins.com/news-and-events/article-1/Redskins%E2%80%99-Dry-Needles-Speed-Recovery-Time/744d3f1e-b8c2-4f5d-8735-c4f8c8a2c468 68. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain.Pain. 2011 Mar; 152(3 Suppl):S2-15. doi: 10.1016/j.pain.2010.09.030. 69. Galer BS, Argoff CE. Defeat Chronic Pain Now. Fair Winds Press; 1 edition (December 1, 2010). |
|||||
| Return to top of page | |||||
| Questions or problems regarding this web site should be directed to DRSTEIN@VASG.ORG . Copyright © 2003 ASAH. All rights reserved. Last modified: April 15, 2014 . |
|||||